Addex Therapeutics (ADXN)·H1 2025 Earnings Summary
Addex Therapeutics H1 2025 Earnings
Executive Summary
Addex Therapeutics (ADXN) reported H1 2025 results highlighting significant pipeline progress despite a lean cash position. The Swiss clinical-stage biotech continues to advance its GABAB PAM programs for chronic cough and substance use disorders while managing a streamlined cost structure post-Neurosterix spinout.
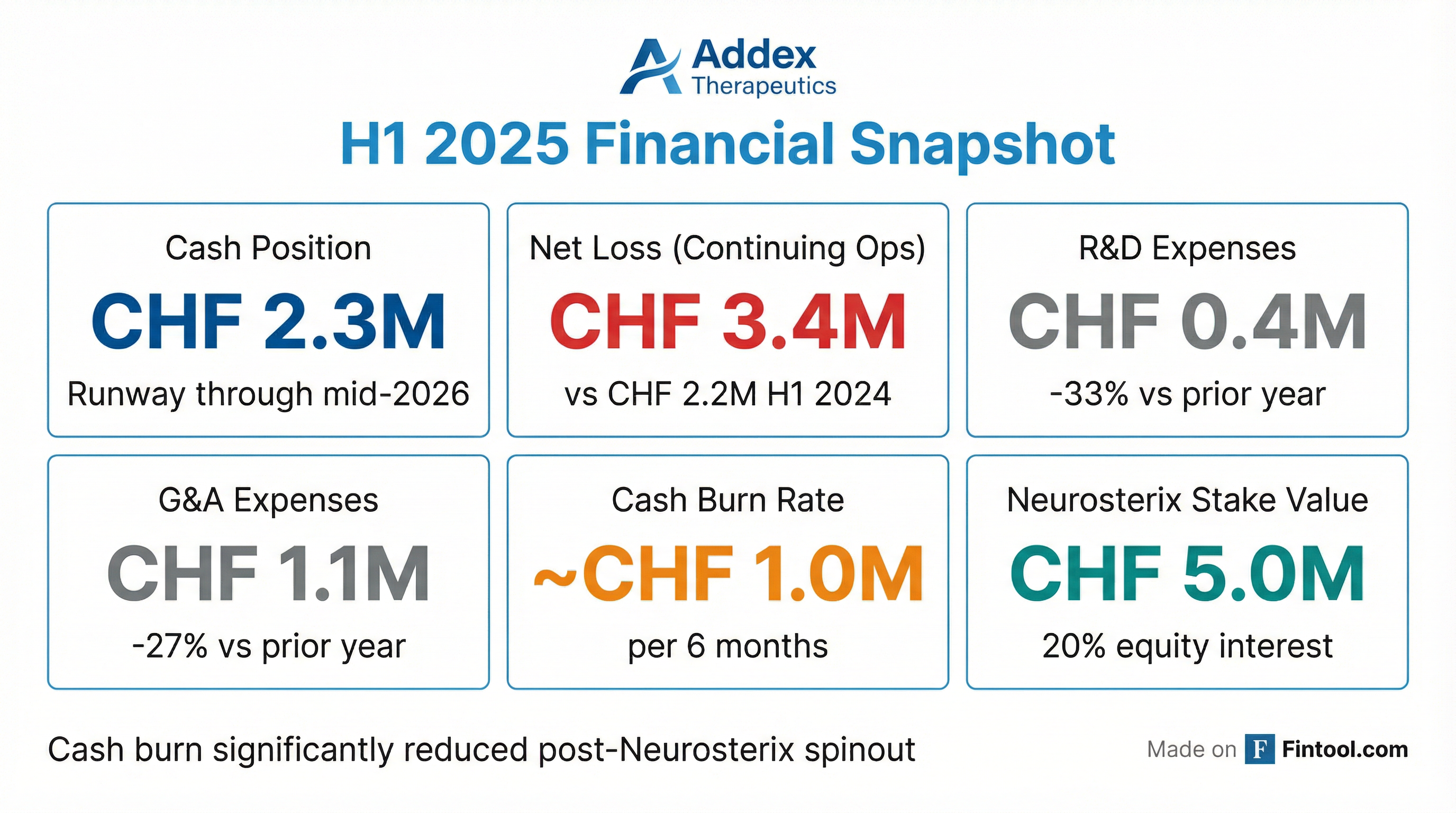
Key Metrics
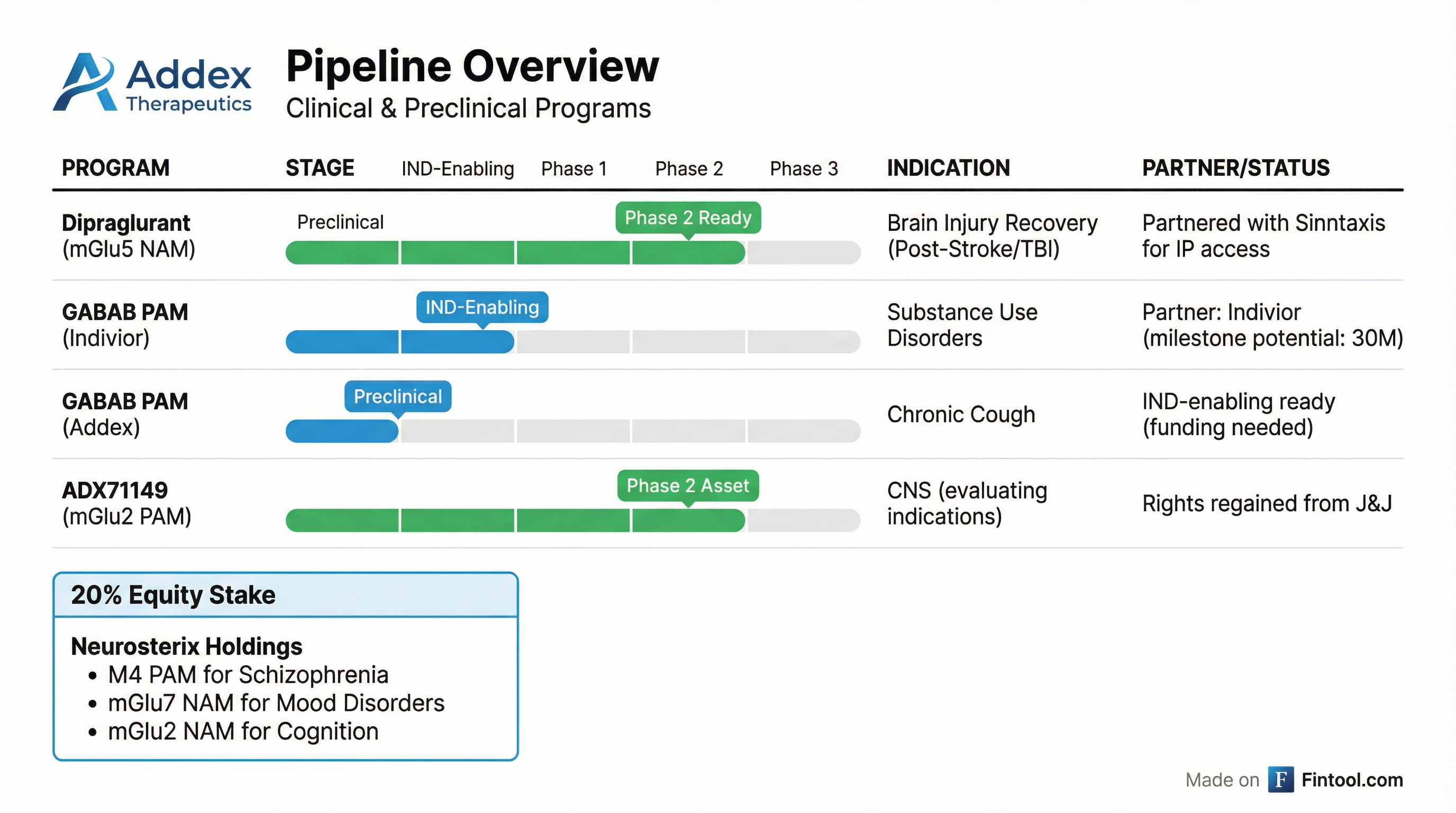
Pipeline Progress
What Went Well
GABAB PAM for Substance Use Disorders (Indivior Partnership):
"Our partner Indivior has selected a GABAB PAM drug candidate for development in substance use disorders and has successfully completed IND-enabling studies."
- Milestone potential: $330 million in regulatory, clinical, and commercial milestones
- Royalties: High single digit to low double digits on net sales
GABAB PAM for Chronic Cough (Addex-Owned):
"We have substantially completed preclinical profiling of our selected drug candidate and recently published robust antitussive data in multiple preclinical models of cough."
- Compound A achieved 70% reduction in cough number at maximal doses
- Superior to baclofen, naltrexone, aprepitant, and codeine in head-to-head studies
- >60-fold safety margin demonstrated based on respiratory depression and sedation
- No signs of tolerance after subchronic dosing
Strategic Investments:
- Invested CHF 795K in Stalicla SA (precision medicine for neurodevelopmental disorders)
- Regained rights to mGluR2 PAM program (ADX71149) from Johnson & Johnson
What Needs Attention
Funding Constraints:
"Current cash does not fund the progression of our unpartnered programs into the clinic."
- IND-enabling studies for chronic cough program subject to securing financing
- Neurosterix stake value declined to CHF 5.0M from CHF 9.4M (equity method losses)
Financial Results
Income Statement (Continuing Operations)
Balance Sheet Highlights
Cash Flow
Stock Performance
Management Commentary
On Chronic Cough Program:
"We intentionally selected a centrally acting compound in order to broaden and maximize the range of chronic cough patients that we can aim at... The progress of nalbuphine nicely captures the potential of centrally acting antitussive drugs and their superiority over peripherally restricted antitussive drugs."
On Competitive Landscape (M4 PAM Space):
"AbbVie have now moved their M4 PAM, emraclidine, back into clinical development. So this is very exciting news... We've also seen Neumora as well moving two compounds into phase one. So I think we and others are strongly believing in the M4 PAM space."
On Neurosterix Progress:
"Neurosterix has made excellent progress with their lead M4 PAM drug candidate successfully completing IND-enabling studies. The program is on track to dose patients this year."
Q&A Highlights
HC Wainwright Analyst on Stalicla and Neurosterix:
Q: Can you comment on recent developments in the neuropsychiatry space that might have implications for Neurosterix and Stalicla?
Tim Dyer (CEO): "It's very encouraging to see that there is continued renewed excitement within the neuropsychiatry CNS space... We spun out Neurosterix with CHF 65 million in financing... really as a financing mechanism to get our portfolio of neuropsych assets moving. And they are moving very, very nicely."
On Chronic Cough Target Patient Population:
Mikhail Kalinichev (Head of Translational Science): "The central approach, the central activity is essential for achieving maximal coverage of a variety of patients within the chronic cough domain."
Tone Evolution: H2 2024 → H1 2025
Management tone: Consistently optimistic about pipeline progress while transparent about financing needs. Focus has shifted from Neurosterix transaction benefits to advancing proprietary programs through IND stage.
Key Takeaways
-
Indivior Partnership Progressing: IND-enabling studies successfully completed; major milestone potential ($330M) intact
-
Chronic Cough Program Ready: Robust preclinical data positions GABAB PAM as potential best-in-class; IND-enabling studies contingent on financing
-
Lean Operations: Post-spinout burn rate significantly reduced (~CHF 2M/year continuing ops)
-
Financing Risk: CHF 2.3M cash with mid-2026 runway; additional capital needed to advance programs into clinic
-
Strategic Optionality: Stalicla investment, ADX71149 rights, and dipraglurant repositioning provide additional value creation opportunities